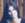im Zuge einer gegenseitigen Vollmacht
English translation: by way of a power of attorney (countersigned/acknowledged by the same)
| GLOSSARY ENTRY (DERIVED FROM QUESTION BELOW) | ||||||
|---|---|---|---|---|---|---|
|
| 13:59 Jul 30, 2020 |
| German to English translations [PRO] Medical - Law (general) / Klinische Prüfungen | |||||||
|---|---|---|---|---|---|---|---|
|
| ||||||
| Selected response from: Stuart and Aida Nelson United Kingdom Local time: 06:20 | ||||||
Grading comment
| |||||||
| Summary of answers provided | ||||
|---|---|---|---|---|
| 3 | appointed as co-sponsor (and recorded on the application form) |
| ||
| 3 | reciprocal authorisation |
|
| Discussion entries: 12 | |
|---|---|
Answers
1 hr confidence: 

appointed as co-sponsor (and recorded on the application form) Explanation: Legal representatives – CTIMPs If a sponsor(s) of a CTIMP is not based in the European Economic Area (EEA), it is a statutory requirement to appoint a legal representative based in the EEA for the purposes of the trial. Details of the legal representative should be entered in the ‘legal representative’ section of IRAS. The legal representative: may in some cases enter into specific contractual arrangements to undertake some or all of the statutory duties of the sponsor in relation to the trial, in which case the legal representative would also be regarded as a co-sponsor and would then require insurance or indemnity cover. Where the legal representative is also a co-sponsor, this should be separately recorded on the application form and details given of the allocation of sponsorship responsibilities. Evidence of insurance or indemnity cover should be provided. https://www.hra.nhs.uk/planning-and-improving-research/resea... Mutual power of attorney would only be applicable to natural persons in case of illness or otherwise, but I am not aware that a mutual power of attorney would be made between companies. In this case, the Legal Representative would probably be a CRO (Clinical Research Organisation). In any event, I would ask the client for confirmation. I hope it helps -------------------------------------------------- Note added at 3 hrs (2020-07-30 17:25:21 GMT) -------------------------------------------------- And maybe it is best to use the literal translation: "by means of/within the process of a mutual power of attorney" and add a question if this is about co-sponsorship. Normally, when applying for approval of a clinical trial, companies have to submit many application forms, use platforms established by the monitoring bodies, etc. -------------------------------------------------- Note added at 23 hrs (2020-07-31 13:19:03 GMT) -------------------------------------------------- Based on further context: If the sponsor is not based in … a legal representative must be appointed. The legal representative must be appointed by way of a power of attorney countersigned/acknowledged by the same. Depending on the context, it can also be that the legal representative has a “Bevollmächtigten” who would also have to sign the power of attorney. Jedem Antrag ist ein vom Sponsor oder seinem gesetzlichen Vertreter oder dessen Bevollmächtigten unterzeichnetes Anschreiben in deutscher Sprache mit entsprechender Vollmacht (in deutscher oder englischer Sprache) beizulegen, das folgende Angaben umfassen muss: https://www.bfarm.de/SharedDocs/Downloads/DE/Arzneimittel/Zu... In addition to the information listed in the DIMDI's template, the application is to be accompanied by the following documents, in German or English as applicable in the individual case, unless expressly specified in the following that submission is to be in German (Section 3 MPKPV): 1. The trial protocol - or, in case of a performance evaluation study, the evaluation protocol - signed by investigator, leading investigator of the clinical trial or the performance evaluation study as well as by the sponsor or appointed representative, 11. a power of attorney for the representative appointed by the sponsor according to Section 20 sub-section 1 sentence 4 number 1a MPG, https://www.bfarm.de/EN/MedicalDevices/ClinicalTrials-MD/Aut... |
| |
Grading comment
| ||
| Login to enter a peer comment (or grade) | ||
1 day 43 mins confidence: 

reciprocal authorisation Explanation: I think this is what you are looking for - the "gegenseitig" isn't "mutual" in this case, but reciprocal. I would use authorisation because power of attorney is too broad in this context. -------------------------------------------------- Note added at 1 day 48 mins (2020-07-31 14:47:14 GMT) -------------------------------------------------- forgot your "im Zuge einer" - I would simply translate that "must be declared in a reciprocal authorisation" or, if you want it a bit fancier, "by way of". Hope that helps. https://www.rcr.ac.uk/sites/default/files/di_irmer_guidance_consultation_draft_29-10-2019.pdf Reference: http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=52E... |
| |
| Login to enter a peer comment (or grade) | ||
Login or register (free and only takes a few minutes) to participate in this question.
You will also have access to many other tools and opportunities designed for those who have language-related jobs (or are passionate about them). Participation is free and the site has a strict confidentiality policy.
KudoZ™ translation help
The KudoZ network provides a framework for translators and others to assist each other with translations or explanations of terms and short phrases.
See also:
Search millions of term translations