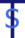driven by chronic inhaled tobramycin
German translation: Tobramycin-bedingt; auf [die] Tobramycin[-Inhalationsbehandlung] zurückzuführen
| GLOSSARY ENTRY (DERIVED FROM QUESTION BELOW) | ||||||
|---|---|---|---|---|---|---|
|
| 15:48 May 22, 2014 |
| English to German translations [PRO] Medical - Medical: Pharmaceuticals / Klinische Studie | |||||||
|---|---|---|---|---|---|---|---|
|
| ||||||
| Selected response from: Anne Schulz Germany Local time: 23:51 | ||||||
Grading comment
| |||||||
| Summary of answers provided | ||||
|---|---|---|---|---|
| 3 +3 | Tobramycin-bedingt; auf [die] Tobramycin[-Inhalationsbehandlung] zurückzuführen |
| ||
| 4 | ausgelöst durch die langfristige Inhalation von Tobramycin |
|
| Discussion entries: 4 | |
|---|---|
Answers
39 mins confidence: 

ausgelöst durch die langfristige Inhalation von Tobramycin Explanation: Einige Kunden verwenden "chronic" anstelle von "long-term". "Driven" ist ebenso ein Begriff, der schwammig ist. Aber das sollte so passen. |
| |
| Login to enter a peer comment (or grade) | ||
16 hrs confidence: 
 peer agreement (net): +3
peer agreement (net): +3
Tobramycin-bedingt; auf [die] Tobramycin[-Inhalationsbehandlung] zurückzuführen Explanation: Nach der Studienbeschreibung unten kommen für "driven by tobramycin" wohl zwei Interpretationen infrage: 1) Tobramycin beeinträchtigt die Wirkung von Ataluren (und ist dafür verantwortlich, dass der Wirkungsunterschied Ataluren vs Placebo bei Antibiotika inhalierenden Patienten weniger deutlich ausfällt), oder 2) die meisten Antibiotika-inhalierenden Patienten inhalieren Tobramycin (das die Wirkung von Ataluren beeinträchtigt). Genauso komprimiert und "vielsagend" wie im Quelltext könntest du beispielsweise sagen: Tobramycin-bedingt fiel der Unterschied Ataluren-Placebo bei [Patienten mit Antibiotika-Inhalation] weniger ausgeprägt aus. Oder: Der Unterschied fiel [...]; dies ist auf die Tobramycin-Inhalation zurückzuführen. "The Phase 3 double-blind, placebo-controlled study, which was conducted across 11 countries, compared ataluren (n=116) to placebo (n=116) in nmCF patients. The primary endpoint, the relative change from baseline in %-predicted FEV1 at 48 weeks, showed a positive trend favoring ataluren versus placebo, and a larger effect in patients not receiving chronic inhaled tobramycin. In the intent-to-treat population, there was a 3% difference in the relative change from baseline in %-predicted FEV1 between the ataluren and placebo groups at Week 48 (-2.5% change on ataluren vs. -5.5% change on placebo; p=0.12) which was not statistically significant. An analysis of the relative change from baseline in %-predicted FEV1 across all post-baseline study visits demonstrated an average difference between ataluren and placebo of 2.5% (-1.8% average change on ataluren vs. -4.3% average change on placebo; p= 0.048). There were 23% fewer pulmonary exacerbations in the ataluren group compared to placebo (p=0.0992). Further results from a post hoc analysis of the subgroup of patients not receiving chronic inhaled tobramycin showed a 5.7% difference in relative change from baseline in % predicted FEV1 favoring ataluren, with a mean change from baseline of -0.7% in the ataluren arm, and – 6.4% in the placebo arm (nominal p=0.0082). In addition, there were 40% fewer exacerbations in ataluren-treated patients in this subgroup. The outcomes observed in multiple endpoints between the subgroup of patients who were not prescribed chronic inhaled tobramycin and the subgroup of patients who were prescribed chronic inhaled tobramycin as well as post-hoc in vitro testing showing the interference of aminoglycoside antibiotics with ataluren activity support the hypothesis that inhaled tobramycin may interfere with ataluren's mechanism of action." Reference: http://www.marketwatch.com/story/ataluren-phase-3-trial-resu... |
| ||
Notes to answerer
| |||